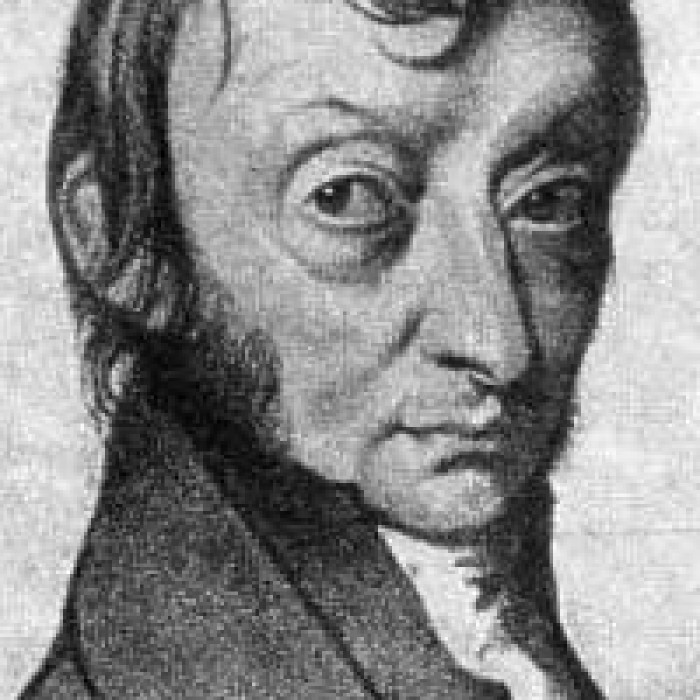
Lorenzo Romano Amedeo Carlo Avogadro, Count of Quaregna and Cerreto (9 August 1776, Turin, Piedmont-Sardinia – 9 July 1856), was an Italian scientist.
His most noted for his contribution to molecular theory now known as Avogadro's law, which states that equal volumes of gases under the same conditions of temperature and pressure will contain equal numbers of molecules.
Avogadro was born at Turin to a noble family of Piedmont-Sardinia in the year 1776. That city, now part of Italy, was then of the Kingdom of Sardinia.
He graduated in ecclesiastical law at the late age of 20 and began to practice. Soon after, he dedicated himself to physics and mathematics.
In 1820, he became a professor of physics at the University of Turin.
Little is known about Avogadro's private life, which appears to have been sober and religious. He married Felicita Mazzé and had eight children.
In honor of Avogadro's contributions to molecular theory, the number of molecules in one mole was named Avogadro's number, NA or "Avogadro's constant". It is approximately 6.0221415×1023.
Avogadro's number is used to compute the results of chemical reactions. It allows chemists to determine amounts of substances produced in a given reaction to a great degree of accuracy.
Avogadro's Law states that the relationship between the masses of the same volume of same gases (at the same temperature and pressure) corresponds to the relationship between their respective molecular weights.
One of his most important contributions was clearly distinguishing one from the other, stating that gases are composed of molecules, and these molecules are composed of atoms.
Avogadro is hailed as a founder of the atomic-molecular theory.
Only through studies by Charles Frederic Gerhardt and Auguste Laurent on organic chemistry was it possible to demonstrate that Avogadro's law explained why the same quantities of molecules in a gas have the same volume.
Johann Josef Loschmidt first calculated the value of Avogadro's number, often referred to as the Loschmidt number in German-speaking countries (Loschmidt constant now has another meaning).
In 1911, a meeting in Turin commemorated the hundredth anniversary of the publication of Avogadro's classic 1811 paper. King Victor Emmanuel III attended. Thus, Avogadro's great contribution to chemistry was recognized.
Rudolf Clausius, with his kinetic theory on gases proposed in 1857, provided further evidence for Avogadro's Law. Jacobus Henricus van 't Hoff showed that Avogadro's theory also held in dilute solutions.
He died on 9 July 1856.
Source: Link

1564 - 1616

1803 – 1882

1854 – 1900

1942 – 2016

1928 – 2014

1835 – 1910

1869 – 1948

1884 – 1962
1898 – 1963

1929 – 1993

1879 – 1955

1809 – 1865

1807 – 1870

1800 – 1859

1795 – 1821

1755 – 1793

1984 -

1989 – 2011

1943 – 2001

1815 – 1902

1929 – 1994
1767 – 1848
